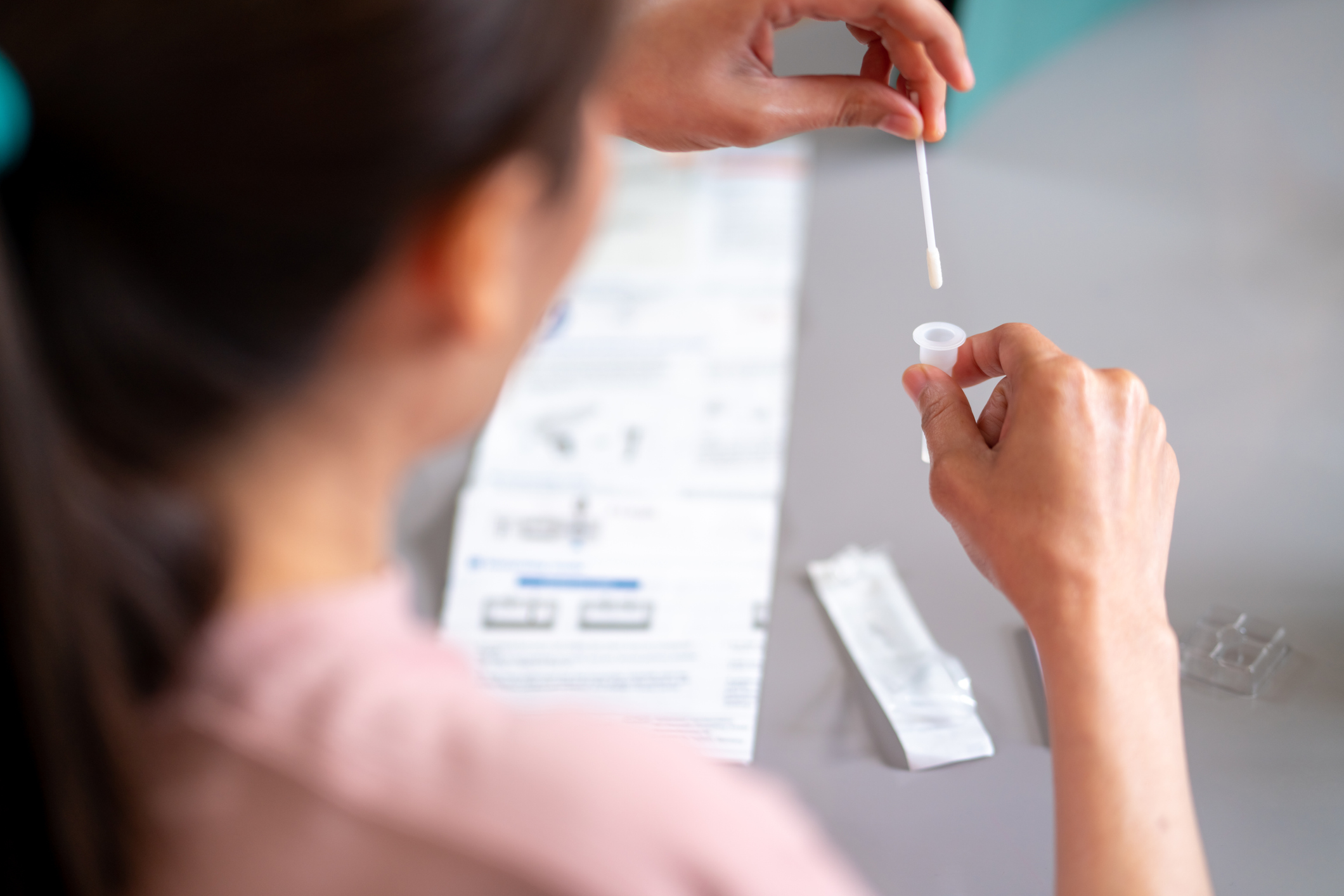
Ice and snowstorms across Canada and the United States this week emphatically demonstrated Winter hasn’t relinquished its reign just yet, and seasonal colds, flu and RSV (Respiratory Syncytial Virus) are still circulating despite trending downward. No home test for flu has been available – until now, as the U.S. Food and Drug Administration on Friday authorized the first combination test for the flu and the coronavirus that can be given at home to determine which pathogen is causing respiratory illness.
According to the Washington Post, the agency granted emergency use authorization to the Lucria Covid-19 & Flu Test, which, using samples collected by nasal swabs, offers results in 30 minutes. This winter, the flu, RSV, and COVID have all been circulating, making it difficult to determine which is responsible for respiratory tract infection symptoms. The Lucira test can be purchased without a prescription by anyone over the age of 14 and the cost reimbursed through private insurance using a receipt.
The arrival of a combination at-home test is good news, helping people get more timely screenings that lead to appropriate prescribing of medication such as Paxlovid for Covid-19 or Tamiflu for influenza. The latest test is just one example of how the pandemic has moved the FDA forward in helping to provide greater access to at-home testing for infectious diseases to support public health.
Patients who take an at-home Lucira test are urged to report their results to their doctor and to access appropriate medical care and treatment. The at-home test may also help prevent the unnecessary use of antibiotics, which don’t work to treat viruses, and that can lead to antibiotic resistance. For those at high risk for serious illness, these tests provide greater access to early care.
The new COVID variant, the XBB.1.5 is a highly transmissible descendant of omicron that is judged to cause about half of new infections in the U.S. Because only about a third of Americans over the age of 65 have received the updated bivalent booster, the new variant is capable of causing serious illness in elderly and immunocompromised people. The CDC is urging people to get the bivalent booster if they haven’t already.




Add Your Voice
0 Comments
Join the Discussion