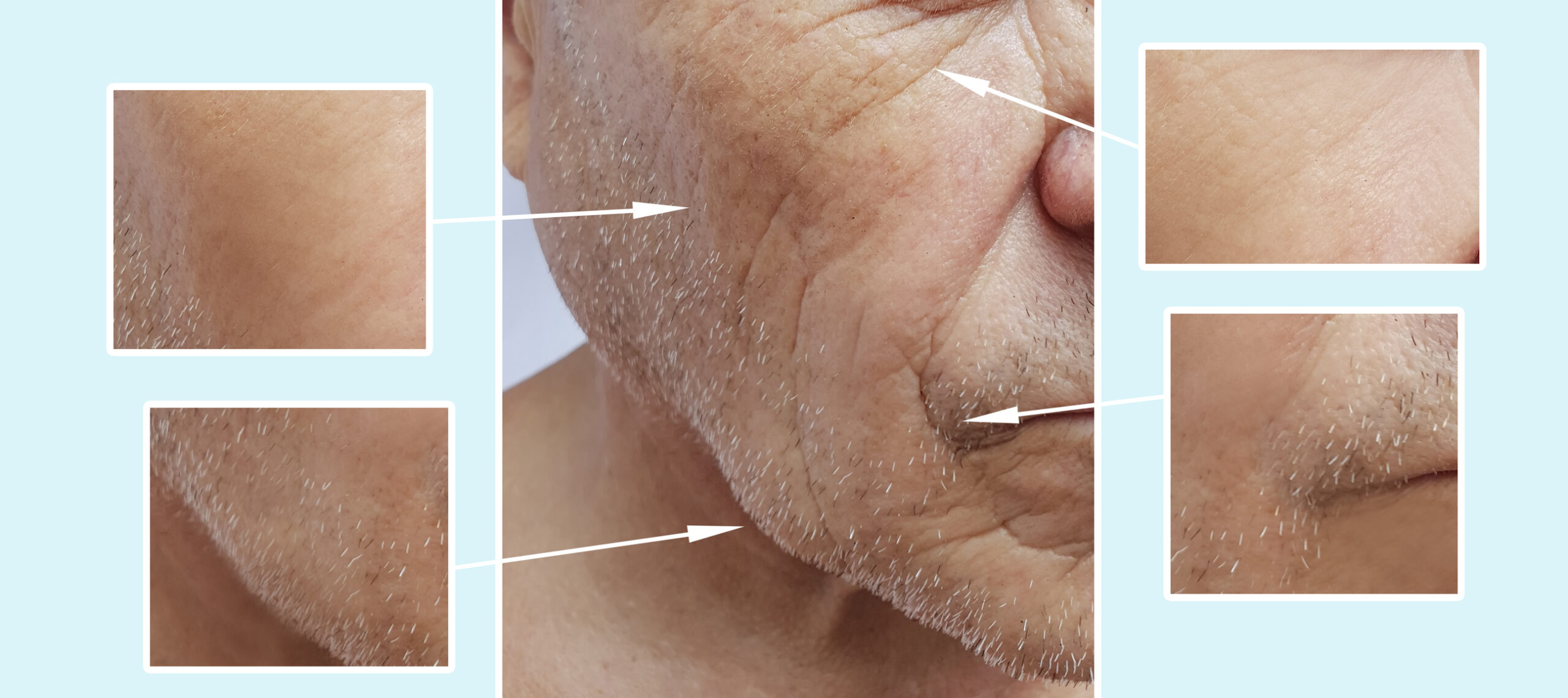
Facial fillers are used to smooth out fine lines and wrinkles and to enhance the cheeks and lips that tend to lose volume as people age. There have been a few incidences of side effects, including among Moderna trial participants who had cosmetic facial fillers. The vaccine triggers the body’s immune system and can cause an inflammatory response in areas where filler has been injected. Mild reactions can be treated by the practitioner who injected the filler but a severe reaction should be seen by an emergency room doctor.
According to The Aesthetic Society, mild localized facial swelling in the three cases reported in the recent Moderna vaccine trial were treated with steroids or antihistamines. There have been no reported cases of adverse reactions associated with facial fillers with the Pfizer COVID-19 vaccine. None of the cases were life-threatening or required the use of an EpiPen or hospitalization.
As with any vaccine, people with a history of allergic reaction should have an EpiPen ready when receiving their inoculation. Having a history of facial dermal fillers should not prevent people from receiving the vaccines; the side effects are minimal and treatable without long-term complications. Being infected with the novel coronavirus is far riskier than receiving the vaccine and having a mild reaction.
In a recent Centers for Disease Control and Prevention report, there have been 21 cases detected of anaphylaxis following the first 1,893,360 doses of the Pfizer-BioNTech COVID-19 vaccine; 71 percent of these occurred within 15 minutes of injection. Most cases had symptom onset within 30 minutes of vaccination among people with a history of allergies or allergic reactions. The majority of the cases occurred in women.
Anaphylaxis appears to be a rare event among recipients of COVID-19 vaccines and the CDC and the FDA are enhancing monitoring as well as bolstering screening and on-site response for any allergic reactions.




Add Your Voice
0 Comments
Join the Discussion